Find out more about how to get iso 13485 certification to understand where your business stands at the moment and what you should begin with.
According to the newest ISO Survey, the global amount of ISO 13485 certificates has grown up to a 33% during the last year. The increase in demands is impressive. So what does the document stand for?
The safety of medical professionals and patients has to be protected by a valuable guarantee. Healthcare suppliers use ISO 13485 in relation to quality workflows control. But its main goal is laying out quality standards for running a medical system where a company has to prove both its competence in providing medical devices and complementary services that always follow clients’ and regulative demands. Your company may need one or several stages for certification such asproject design and development, management, delivery and storage, launching, or medical product service, or provision of related activities.
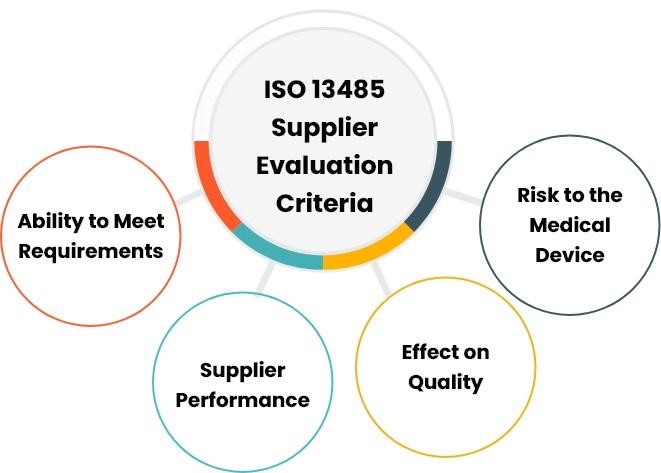
ISO 13485 requirements are not a piece of cake to accomplish. To get the desired certificate means your business is able to meet high-quality standards in services. Let us see the main stages and how to do your best at each step you are involved in.
The quality system philosophy
The requirement to plan quality goes first and finding an experienced ISO consultant is a must-do here. In fact, you can have several certification agents, one for each of your medical setting locations, if you have plenty of cash. Still, choosing one consultant for all of your locations is more practical in terms of time and budget. To select an ISO partner, first, you have to submit an application form and call for a quote.
It is not enough to just sit and compose a quality reference book together under the expert’s guidance. A documented quality scheme is needed for carrying out changes to your quality control policy. You are allowed a free format for your quality plans, we only mention Gantt Charts and spreadsheets as the most popular ones.
During the next two steps, you are going to drive your effort towards following all regulatory requirements and also deal with design controls implementation. Quality plan development in the USA medical market must be FDA 21 CFR 820 compliant.
Support, Core and Management processes
In this section, you will learn what the traditional pattern of a process interaction figure looks like. You must demonstrate this synergy in your quality manual because it is an important requirement for ISO 13485 certification.
Document monitoring, guided skills practice and other support routines make one level. Shopping, producing, and delivery processes which are basically the core ones, are put above it. And the final top level is for management tasks.
Each of these rows does not come alone but with affiliated activities, and they have to be monitored. So, it is most practical to write the document control procedure at first because it will play the role of foundation for the whole system.
After the approval of all possible procedures referred to the record, document and design controls, the right time comes for registering training. It is important to divide this report into three paragraphs – practice, its success and expertise. Once it’s done, you can move to the rest of the procedures, which are less than 20 according to ISO 13485 plus a couple of additional ones depending on the location and national laws.
With time, as you fill in the complementary sections, your manual will be bigger and bigger, showing what you do truly and chronologically – contrary to copy-and-paste the data from the standard. In fact, by keeping this kind of “diary” – writing the procedures steadily – you will have the quality manual 70-80% complete. And that will serve you so well, you will see.
The Management Processes Stage
Internal Auditing, CAPA and Management Review are considered to be the prime ones. Usually, it is internal auditing first, where some weak points are spotted, as a rule. CAPA provides curative and precautionary actions here.
A Management Review is quite simple – writing a page of strict standard requirements will not take much time and effort.
Finally, with the help of an independent expert, a final audit will be conducted either in person or remotely. That means you are ready for Stage 1.
Reaching the top. The Certification Audit
This one is performed in two stages. First, your company will be reported of what the good and bad discoveries were made during the day of investigation. Consequently, the negative findings will have to be resolved during Stage 2. It will take more than a month ideally, and additional auditors may be needed. The final certificate is a reason to celebrate with champagne indeed. It means you have done a good job and the reputation of your business and clients’ trust are things to be proud of.
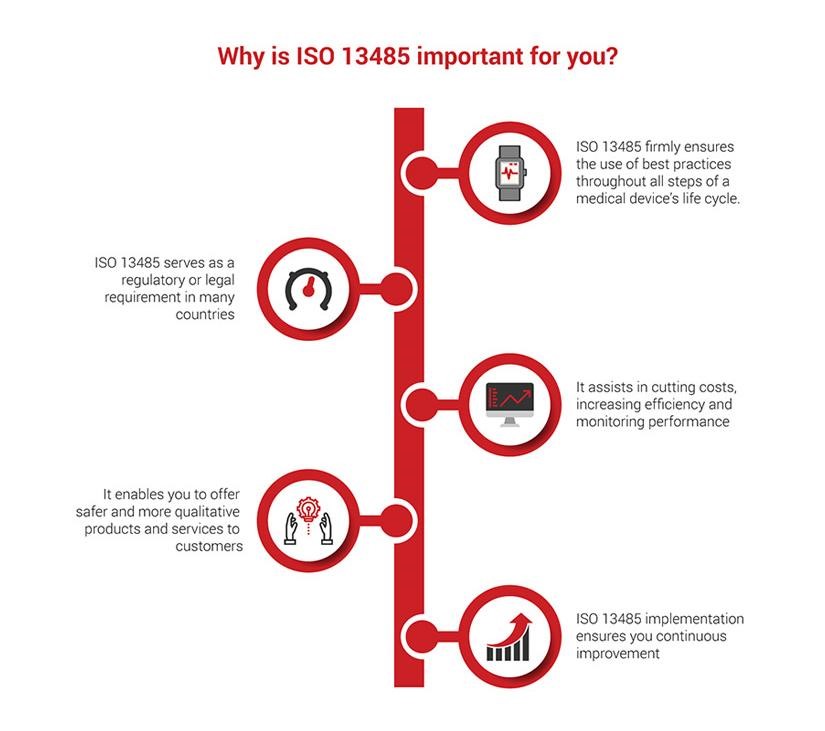

By the way
Here are some things about ISO 13485 certification that are worth mentioning.
- It is not a community. A company cannot “join” ISO 13485 standard unless required steps are done to become certified.
- Two employees or two thousand – the size of your company has no role in this document at all.
- It is not a standard for individuals. Only a company or an organization can run this certification process.
- ISO 13485 standard has nothing to do with products’ quality. It is all about the workflow, how well you can manage them, how competent you are and how effective your company can function in general.
ISO 13485 certification is a very important protective measure that makes patients and medical staff trust their healthcare institution and feel safer in terms of service quality and personal data protection.
Our development team has a broad knowledge of medical devices concepts to fit ISO 13485 standards. It is valuablefor avoiding risks, raising efficiency and your business market growth.We know how to plan reviewing and developments processes, minimize expenses, increase effectiveness and guide performance. Implementing phases of the ISO 13485 in your company will help your business follow legal demands and make customers happy. Call us for more details now!






